↑ This 5 minute video is an abridged version of the article below. ↑
A history of Beat Childhood Cancer’s genomic-based treatment for childhood cancer, and a new paradigm for today.
If you follow the clinical research world, you’ve heard of personalized medicine, or precision medicine, or molecularly guided therapy (MGT), or genomics. They’re different terms for what is generally the same concept — personalizing treatment per patient based on specific genetic or molecular analysis. In childhood cancer, genomics is being used to beat cancer on a kid by kid basis.
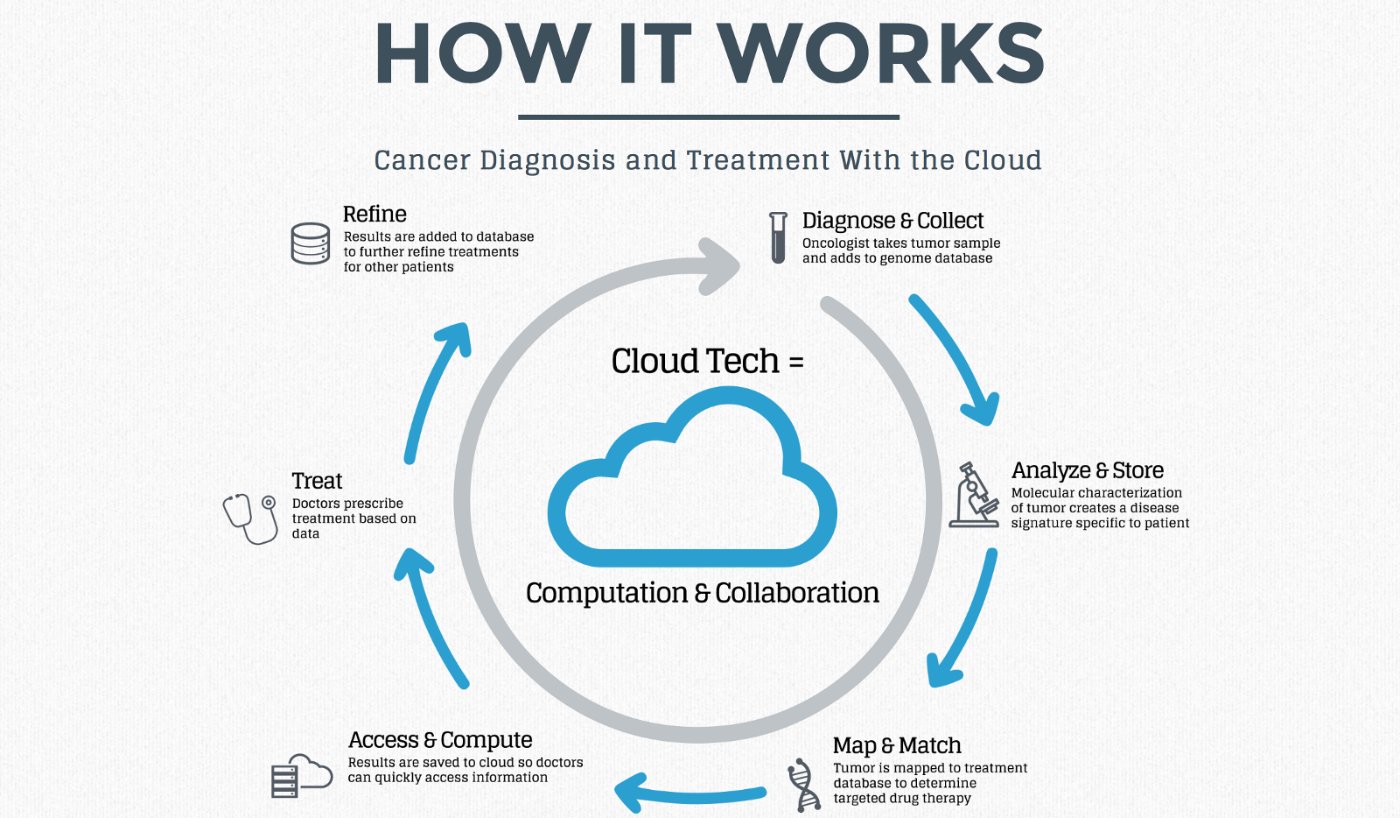
Source: Forbes.
Beat Nb is dedicated to finding and funding a cure for neuroblastoma and other types of childhood cancers. We have directed over $5 million directly to patient-affecting childhood cancer research.
We helped to found, and now fund, Beat Childhood Cancer, an international consortium of 45 hospitals (and growing) conducting clinical trials and research with a shared goal to ours — beating neuroblastoma and other childhood cancers. Much of Beat Childhood Cancer’s work, in partnership with Dell and TGen, has been in leading genomics research in childhood cancer. (Full disclosure: this author sits on the Executive Board for Beat Childhood Cancer, and Beat Nb supports various Beat Childhood Cancer trials financially.)
Some background on genomics: the human genome was first completely mapped in 2003. It took 10 years, and $1 billion. Today, in 2015, TGen can process a patient’s genome in 6 hours. The cost is in the thousands. The sharply dropping costs and speed of genomics have opened a new world of research. There is much work to be done still — we could map the genome in 2003, and now can do so quickly and cost effectively, but what do we do with that data? That is what is being researched worldwide now — across many malady types.
Genomics in cancer is exciting. Cancer kills because a group of cells are mutated, and keep duplicating without stopping. Your body is supposed to maintain a state of equilibrium (called homeostasis) as you go about your life. Cancer cells ignore this, and keep duplicating. The mutations that make cancer cells keep going are what cancer genomics look at. And the goal is to then treat with a drug targeted to that specific mutation — knocking out the tumor with a precise medicine, rather than a blanket chemo hoping to destroy the tumor.
While sequencing can be done at most hospitals today, it is important to understand what depth of sequencing is done for each child, and how this information is being interpreted. Beat Childhood Cancer’s molecular tumor board has developed significant expertise over the last 5 years in the analysis of the genomic sequencing, to make the most informed clinical decisions for children. This is truly the key to best personalized care, taking into account the previous medical treatments, the current condition of the patient and wishes of the family.
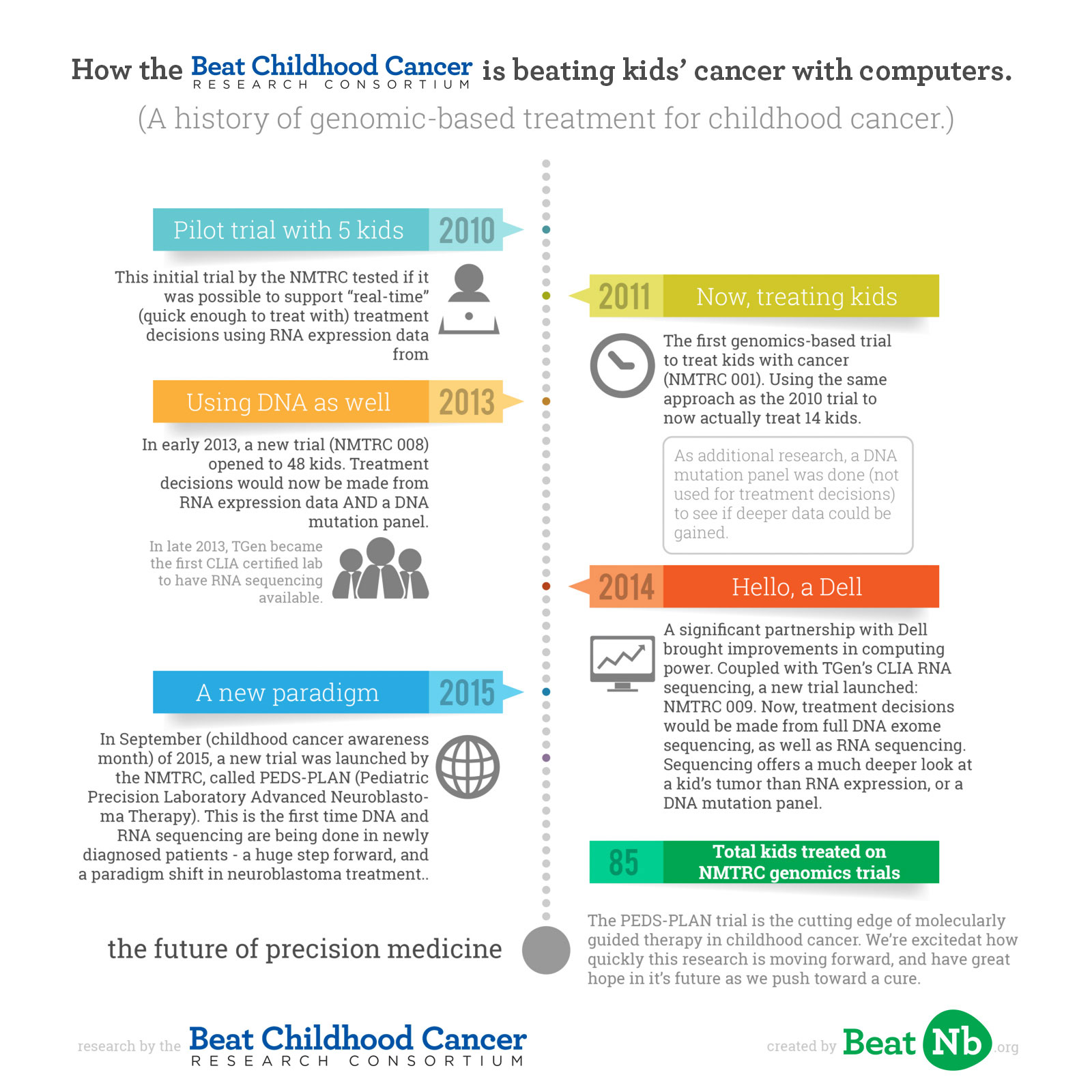
Beat Childhood Cancer, chaired by Dr Giselle Sholler, first started clinically looking at genomics in childhood cancer with a trial opened in 2010. They’d noticed in treating children with neuroblastoma since 2005, kids all had very different tumor profiles. With genomics starting to be clinically relevant, a trial idea was formed.

Dr Giselle Sholler, Chair of Beat Childhood Cancer, and one of her patients. Photo credit: Spectrum Health.
The pilot trial (NCT01109238, completed), opened in April 2010, enrolling 5 children to test if it was possible to support “real-time” (quick enough to treat with) treatment decisions through mRNA expression data genome-wide in neuroblastoma biopsies. At the end of that trial, the process was able to be completed in 12 days. This was the first genomic-based personalized medicine childhood cancer trial in neuroblastoma. (Results published here). It’s important to note, the most accurate and deepest data for determining best treatments comes when using both RNA and DNA expression and sequencing. However, in 2010 the tech wasn’t yet able to support doing all of that quickly enough to get “real-time” data for making treatment decisions. But RNA expression could be done quick enough, so it was time for a new trial, one actually treating kids.
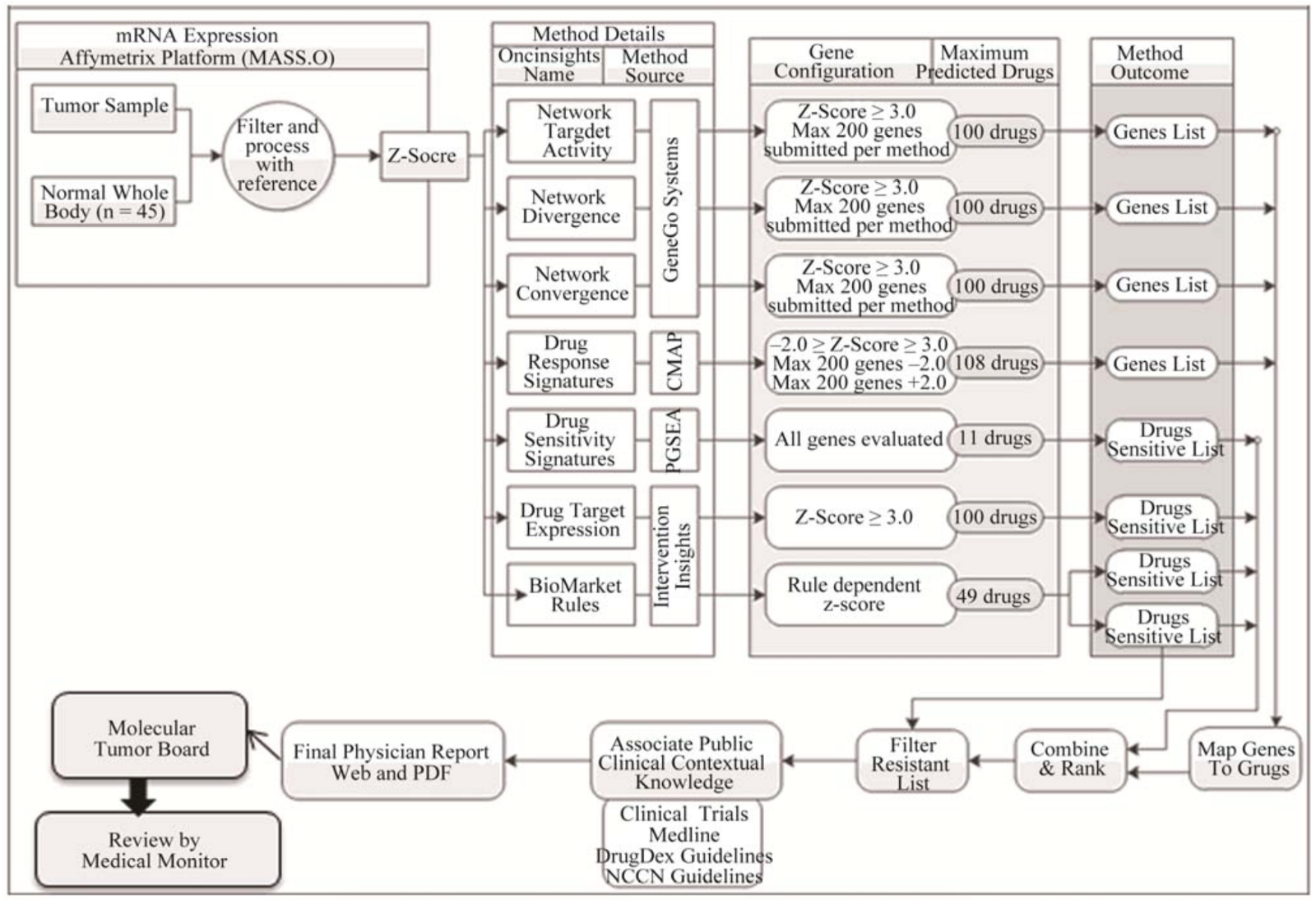
How a drug prediction report was generated in the NCT01109238 study. Source: Journal of Cancer Therapy, 2012, 3, 602–612
The following year, in 2011, a Phase I trial (NMTRC 001, NCT01355679, completed) opened with a larger enrollment of 14 children fighting neuroblastoma, to test the feasibility and safety of actually treating kids with these individualized therapies. (Results published here). Now, Beat Childhood Cancer was treating children using the data generated by their RNA expression profiles (what the first trial tested if was possible). In addition, as research, DNA mutation panels were done (these look at 50 common DNA mutations for cancer) to see if they’d be feasible / useful to add to the data gathered.
A significant partnership with Dell (along with an initial commitment of $4m, which has continued to grow over the years), brought Dell’s cloud supercomputing power to help analyze the data.
In May of 2012, we had written a blog update about the rapidly increasing speed and relevance of using genomics in realtime treatment, after discussions at the 2012 NMTRC Symposium:
This year focused heavily on personalized medicine trials, or targeted treatments. To explain, a little history — chemotherapy, which was only discovered in 1942, is the main treatment for cancer. Chemotherapy is poison — in fact, the first chemo was mustard gas, which was injected into patients after folks during World War 1 noticed it suppressed blood production. Because cancer grows faster than normal cells, the chemo kills cancer faster than it kills the body (is the hope), and then the body recovers (is the hope). You know all those pictures you see of bald, skinny, hollow eyed cancer patients? That’s not cancer doing that — it’s chemo. Of course, it’s the best we’ve had until now.Since mapping the human genome, we can now attempt to target therapy. The NMTRC’s work is focusing on (among other things — and this is a high level overview) taking a biopsy of a tumor, identifying it’s mutations, and treating with a chemotherapy [sic — this could be various drugs] which will target ONLY these mutations — making the treatment go from systemic (poisoning the entire body) to targeted (poisoning only the tumors). It’s showing a lot of promise.
There’s a LOT of information, and up until recently it could take 17 days from biopsy to a list of targeted drugs (these are FDA approved drugs). Dell has stepped up this year to a long-term commitment to the NMTRC’s work with millions of dollars of hardware and systems analyzing. This has already cut over 2/3 of the processing time down — from 17 days to roughly 5 days.
Building on the past three years of research and continuing at, for the clinical research world, a rapid pace, a third personalized medicine trial by Beat Childhood Cancer (NMTRC 008, NCT01802567, ongoing, but not recruiting participants) was opened, accruing 48 kids. The partnership with Dell suggested the hope to complete an RNA expression profile, a DNA Mutation Panel, genomic analysis and report generation, a tumor board held with treatment decision, and treatment review completed and start of treatment — all within 21 days of a child’s biopsy/surgical resection date. It worked.
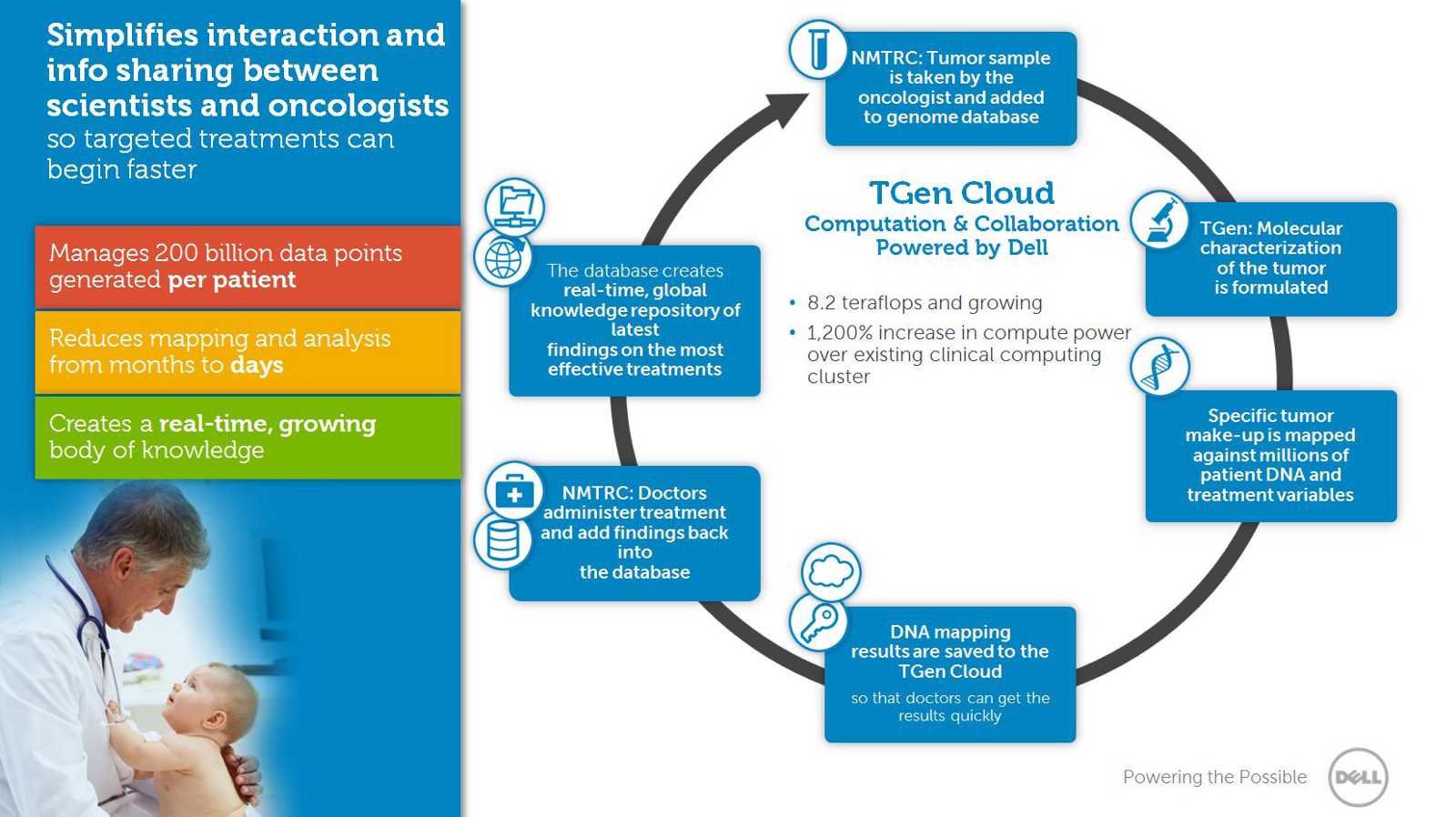
Source: Dell.
Between 2013 and 2014, Dell continued to increase technology and speed, and where sequencing an entire DNA exome (important because it tests allyour DNA mutations, not just a common set) used to take 2 months (not fast enough to be used while treating children, or “clinically relevant”), it could now be completed in 2 weeks. Additionally, TGen became the first CLIA certified lab to have RNA sequencing available, in September of 2014. CLIA certification is needed to use RNA sequencing for treatment decisions. These major advances led Beat Childhood Cancer to open a new trial.
In 2014, a 4th trial was opened (NMTRC 009, NCT02162732, currently recruiting participants). 48 children with cancer would be the goal to test again, now with full DNA exomes being completed, as well as RNA sequencing (remember, sequencing is a much deeper look than expression profile). This study would determine feasibility of using tumor samples to assess genomic sequencing using predictive modeling to make real-time treatment decisions for children with relapsed/refractory cancers. The entire DNA exome was now being sequenced (more powerful than a DNA mutation panel alone), RNA was sequenced, and the data was analyzed and a treatment decision made.

Watch this video to see how TGen and Dell are collaborating with Dr Sholler and Beat Childhood Cancer to beat kids’ cancer.
A new paradigm in treating children with neuroblastoma.
In 2012, the ASCO Post wrote:
Although Dr. Sholler’s trials are the first attempt in personalized medicine for children with relapsed disease, if successful, genomic-based medicine for pediatric patients with cancer may eventually be used in the front-line setting, where the greatest chance for cure may be possible.
Today, that goal is being realized. Just 5 years after Beat Childhood Cancer began clinical trials to research moleculary guided therapy in childhood cancer, in September of 2015 a new trial opened incorporating MGT into upfront therapy (PEDS-PLAN, NCT02559778, currently recruiting participants). This trial represents a new paradigm in treating children with neuroblastoma, based on and uniting the work Beat Childhood Cancer has done with personalized medicine and a drug called DFMO, which hopes to stop neuroblastoma relapse. The trial, called Pediatric Precision Laboratory Advanced Neuroblastoma Therapy (PEDS-PLAN), suggests using molecularly guided therapy in combination with current standard therapy (COG protocol), followed by maintenance therapy with DFMO in subjects with newly diagnosed high risk neuroblastoma.
This is the first time DNA and RNA sequencing are being done in newly diagnosed patients.
A targeted agent (drug) is added to standard of care therapy. Because it is known from experience the expression profiles of tumors change throughout a child’s treatment, they do the RNA and DNA sequencing again after the 4th cycle of chemotherapy, when the tumor is removed. Using precision medicine in this way, in upfront therapy, truly gives the greatest chance for a cure. It’s expensive — full exome sequencing currently costs around $12,000, and RNA sequencing around $7,200. Dell has graciously committed to covering these costs during this trial.
Genomics is an exciting weapon in battling disease, and precision medicine has an army of advocates moving it forward. Not the least of these is thePrecision Medicine Initiative launched in January of 2015 by President Obama. The Precision Medicine Initiative’s mission is to enable a new era of medicine through research, technology, and policies that empower patients, researchers, and providers to work together toward development of individualized treatments.
Others are also carrying this work forward, even in childhood cancer. St. Jude and Washington University have launched the Pediatric Cancer Genome Project. Columbia University Medical Center is now sequencingevery pediatric cancer patient’s tumor. As the NMTRC marks five years into childhood cancer genomics research, more continue to join the battle.
A new trial, the NEPENTHE trial (Next-generation PersonalizedNeuroblastoma Therapy) is slated to begin in 2016. However, this trial is using only a DNA mutation panel for the results, not full exome sequencing. Their RNA sequencing is not being done in a CLIA certified lab, and therefore won’t be used to make treatment decision — critical in pediatrics, given the low mutation rate. Scientifically, this study will answer the target question for those drugs, but a concern will be how well these single or double agent treatments without chemo will affect relapsed neuroblastoma.
Collaboration
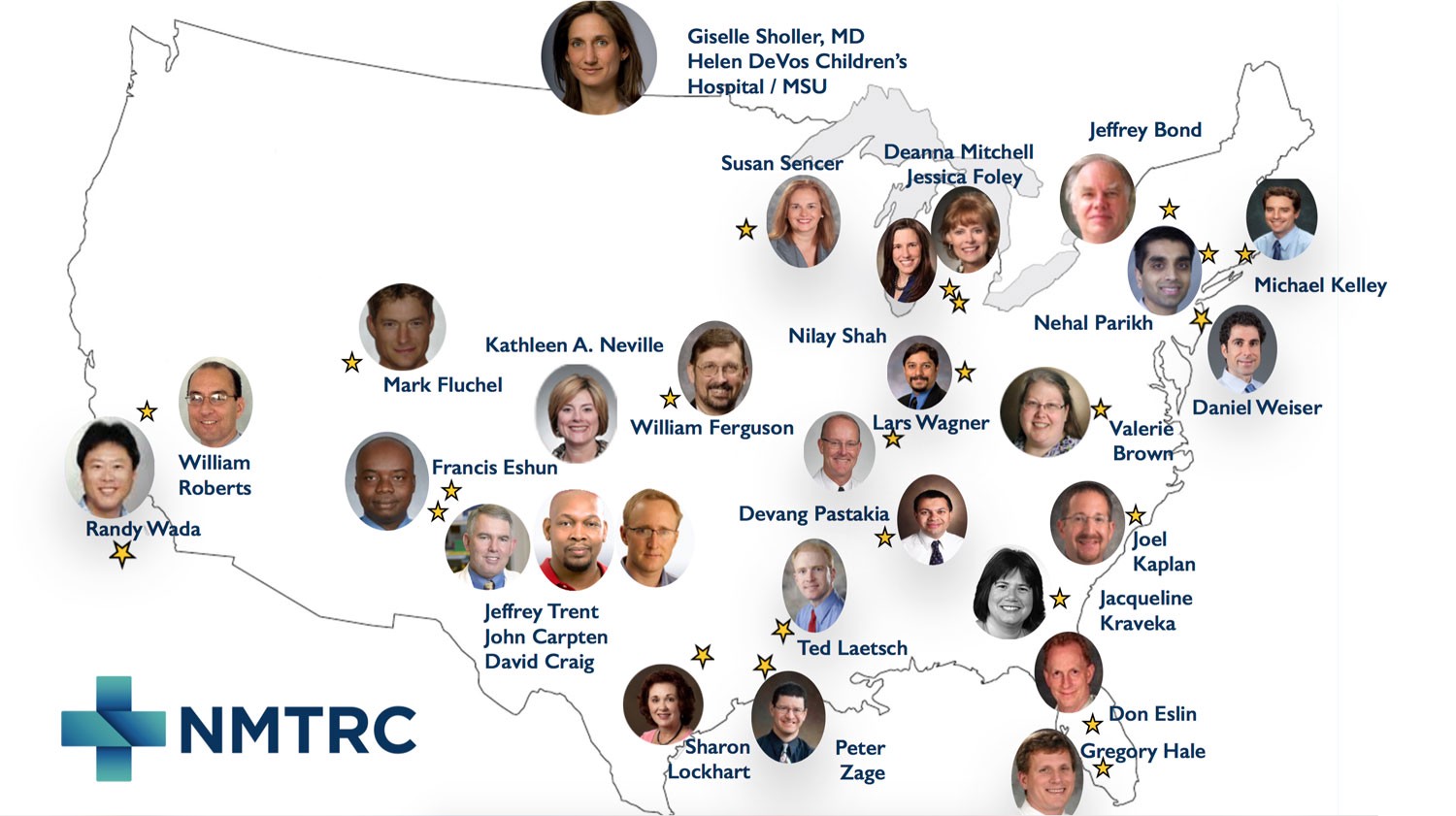
Collaboration is crucial to moving work like this forward. Beat Childhood Cancer now has trials operating in over 25 institutions across the US and internationally. Partners like TGen in Phoenix and Dell in Austin add to the passion and expertise being directed to beating childhood cancer with genomics.
Precision medicine generates vast amounts of data. Converting that data into actionable (and effective) clinical outcomes is a very nuanced and learned approach. The physicians and scientists in Beat Childhood Cancer continue to learn how best to make use of these new approaches, having worked through the process over 80 times now (data, rules, tumor boards to determine best treatment options).
Mature rules have been developed to determine what pathways and drugs are clinically relevant in neuroblastoma. Technical partners are refining analytics and sequencing processes, and speed keeps increasing. Advances in mobile and cloud computing will continue to offer more relevant and available data, and easier ways to digest it for use in the clinic (treating kids). The next five years are just as exciting as what’s happened already.
I wish Ezra could have been diagnosed today instead of in 2009. And of course I don’t mean that — if we’re granting wishes I’d wish he never experienced a single minute knowing what cancer feels like. I can’t help but feel proud, and energized, seeing the huge leaps in treatment and research in the 6 years since Ezra was diagnosed with stage 4 neuroblastoma. Robyn and I know in some small way, Ezra’s name will one day be one of so many whose stories led to a cure. With incredible and caring physicians and resaerchers like those in Beat Childhood Cancer, and other passionate advocates like ourselves, we will beat neuroblastoma.
If you’d like to be a part of this work being done to cure childhood cancer, we welcome your involvement. If you are a parent seeking information on any of these clinical trials (or anyone else with a comment), you can reach us at beatnb.org/contact. You can support financially at beatnb.org/give. Beat Nb is a 501(c)(3) tax-exempt organization with the Internal Revenue Service. Our EIN is 27-2314549.

↑ Ezra. Read more of his story.
Mind if we hang out
in your inbox?
We only email once or twice a month, always relevant to how we're working to beat childhood cancer for every child, everywhere.
Thank you for subscribing!
Something went wrong.
