We drive patient-affecting childhood cancer clinical trials, expanding how research is done.
Precision medicine is the future of cancer treatment. We are helping kids now with targeted therapies and oral drugs with low side effects aimed at preventing or treating relapse without punitive, long-term side effects.
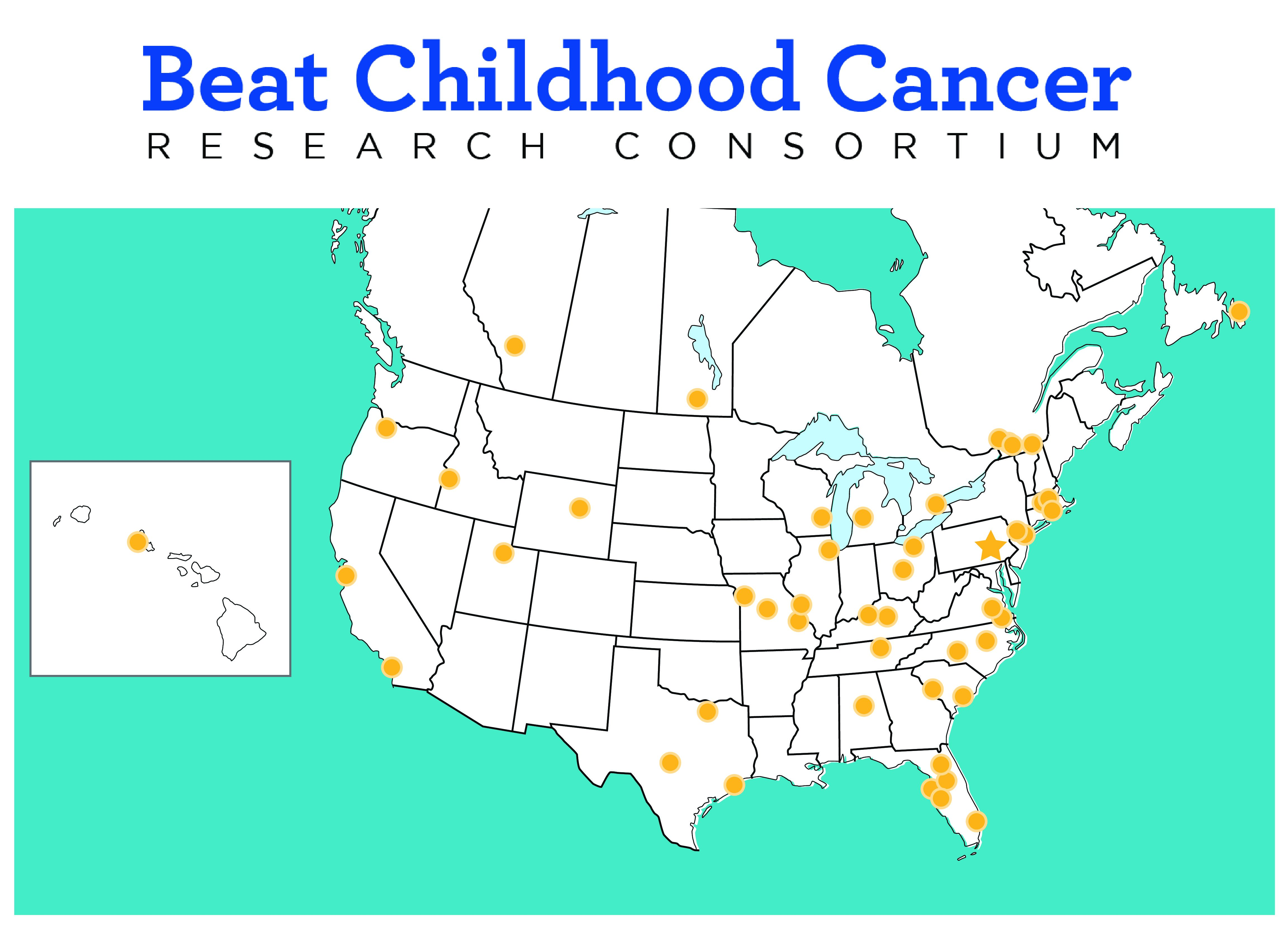
Our community of clinicians, scientists, parents, and supporters has built an international infrastructure and team of research and clinical collaborators to ensure kids have access to these therapies. The Beat Childhood Cancer Research Consortium is made up of over 50 children's hospitals and research institutions in North America.
An active model bringing new treatments to kids today.
Since 2010 we have been developing and expanding a precision strategy to bring precision medicine to kids, while actively creating new treatment options for kids battling cancer.
What we are doing is working. Our group has opened over 24 clinical trials, directly affecting more than 2,000 kids. Where the traditional clinical trial model often takes several years to start enrolling patients, the Beat Childhood Cancer Research Consortium usually enrolls children on clinical trials within one year.
Helping every child, everywhere by creating options.
Our patient-centered focus is not based on a single doctor, hospital, or drug. It is about helping every child, everywhere survive and thrive following a childhood cancer diagnosis.
Every child’s tumor is different; precision, targeted therapies are the future of cancer treatment. By offering genomic sequencing to children diagnosed with cancer at multiple points along the treatment pathway, we:
- gain a greater understanding of what is causing each tumor to be cancer;
- add targeted treatments to current standards of care, directly attacking individual tumors;
- discover novel treatments based on already FDA-approved drugs; and
- work to beat childhood cancer.
