Neuroblastoma (NB) is the most common solid tumor found outside of the brain diagnosed in kids. Two years of age is the average time when a child is diagnosed, and about half of these kids are diagnosed with high-risk (HRNB), which has a very poor prognosis. And sadly, this is despite very intense treatment given to these toddlers to treat HRNB. This treatment would typically include 5-8 cycles of induction chemotherapy, surgery to remove as much of the tumor as possible, one or two cycles of high dose chemo - that can only be survived by receiving an infusion of stem cells given to you while you remain in isolation. This is then followed by radiation therapy, anti-GD2 antibody (which is very harsh) plus cis-retinoic acid therapy. This is a grueling regimen with multiple modalities which can on their own be fatal, and the entire process creates a whole host of intense short and long term side effects.
For children who make it through all of this therapy and are somehow still in remission the fact is that survival numbers (5 years out, when these kids are likely 7 years old) have not improved significantly in the past 20 years.
For those kids who did not respond to initial therapy - or who relapsed following front-line therapy - if they were fortunate enough to respond to additional interventions and get into remission a second time, sadly they have a high rate of another relapse. Generally 80-90% of these kids will relapse again within 2 years, and there is no known curative therapy to treat these children with.
It is clear this is a patient population in desperate need of new therapeutic options. And given the heavy treatment burden that comes along with the current upfront standard of care, there is no room left for additional toxic therapy.
This is the current bleak landscape for kids battling this deadly cancer. We share this backdrop of the natural history of this disease in order to provide the context to properly highlight the recent breakthrough for this patient population reported this year.
A paper titled “Maintenance DFMO Increases Survival in High Risk Neuroblastoma” was published on September 27th 2018 in the Nature Journal “Scientific Reports” about a phase II clinical trial. A drug called DFMO was given to patients in remission from HRNB and the results were not only encouraging but frankly rather amazing. In summary the study authors found:
“DFMO targets a novel pathway and therefore is likely to provide a novel therapeutic strategy for maintaining long-term remission in children with high-risk neuroblastoma. DFMO given to children with high risk NB following completion of either standard multimodal therapy or salvage therapy for relapsed/refractory disease was both well tolerated and appeared to improve the event free and overall survival rates when compared to historical controls.”
This is such a remarkable result because a very well tolerated single agent drug was shown to increase survival in kids with HRNB. Not only did it increase survival, but it is an oral medication that can easily be taken by toddlers. And in an even more astounding twist, almost 70% of the kids on the study reported NO side effects at all. When compared to the treatment these kids are forced to endure for a chance to even get into remission - and to see their extraordinary quality of life while on this maintenance therapy for two years - it is hard to reconcile that both side effect profiles are being used to treat the same disease.
Maintenance therapy is something that most childhood oncologists are very familiar with in other types of childhood cancer - with leukemia being the best example. However, the idea of an extended maintenance therapy for NB is not something that has historically be done. As mentioned above this drug was given to kids for two years who were in remission from NB.
Let us take a minute to answer the question: Why?
There are two very simple reasons:
- If you have NB and are able to achieve remission after finishing initial treatment: 34% of these kids relapse within 2 years and 41% will relapse by 4 years. Sadly the figures also show that 14% of these kids will be dead within 2 years and the number of kids killed by this disease doubles by year 4 when 28% of those kids will be dead.
- The numbers are even more sobering if you had your disease relapse and were then able to get back into remission as 94% of those kids relapse within 4 years and 80% of those kids will be dead within 4 years.
Simply getting kids with HRNB into remission is not enough - while some kids do initially respond to this harsh therapy, the response is not durable. It is a very dynamic and fast growing tumor which may at times respond to traditional cytotoxic chemotherapy. However, biologically there is something driving HRNB tumor growth that is not being addressed with the current therapies utilizing high dose chemotherapy, radiation, immunotherapy and transplant.
The idea behind this study was that perhaps DFMO’s unique targeting of the NB cells - identified in a phase I trial used to treat relapsed and refractory NB - could help to keep kids in remission. NB parents and doctors do not say “my kid is in remission” they instead say “my kid is N.E.D. (no evidence of disease),” because they all know clear scans do not tell the story of this disease. There is a cancer cell population in NB that does not show up on scans but it is clearly there - and perhaps a 2 year maintenance therapy targeting those cells could finally lead to durable remissions for these kids.
So that is what this trial set out to do in two groups of patients. The first group of kids this study analyzed were 100 kids who enrolled on this maintenance trial after having completed upfront therapy and were still in remission.
These kids took DFMO twice a day for two years and the results showed:
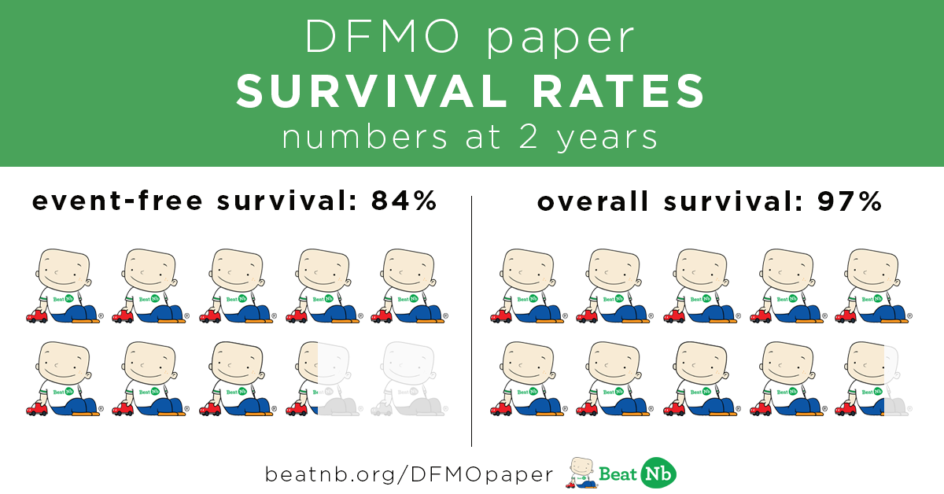
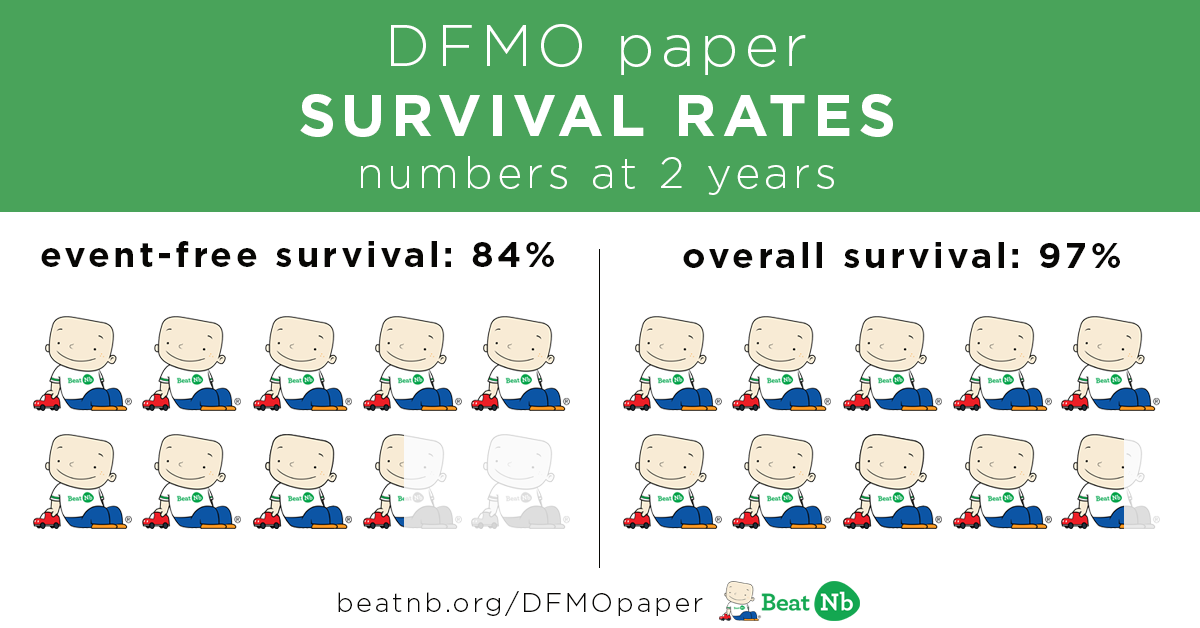
84% of these kids STAYED in remission after 2 years
97% of these kids were still alive after 2 years.
Even more remarkable was the fact that:
At 4 years 83% of these kids were STILL in remission and
At 4 years 96% of these kids were STILL alive.
These percentage increases would be amazing even if they were just being seen at the two year mark. However, the truly breakthrough results coming from this paper are that these results appear to be inducing a sustained remission never previously reported for this disease.
The second group of children this study analyzed were 39 kids who had achieved a remission after having previously relapsed or not responding to upfront therapy. As mentioned above this is an especially difficult patient population that historically has been deemed ‘incurable.’ The results here were also remarkable - and even keeping in mind the smaller number of patients viewed here - these results are eye opening given the historical rates of relapse and death for this patient population. This is a heavily pretreated population who not only had to endure the harsh upfront therapies but also had received various relapses therapies - with this group having a mean of 3.3 previous relapsed therapies and and overall mean time from diagnosis to enrollment of 3.4 years.
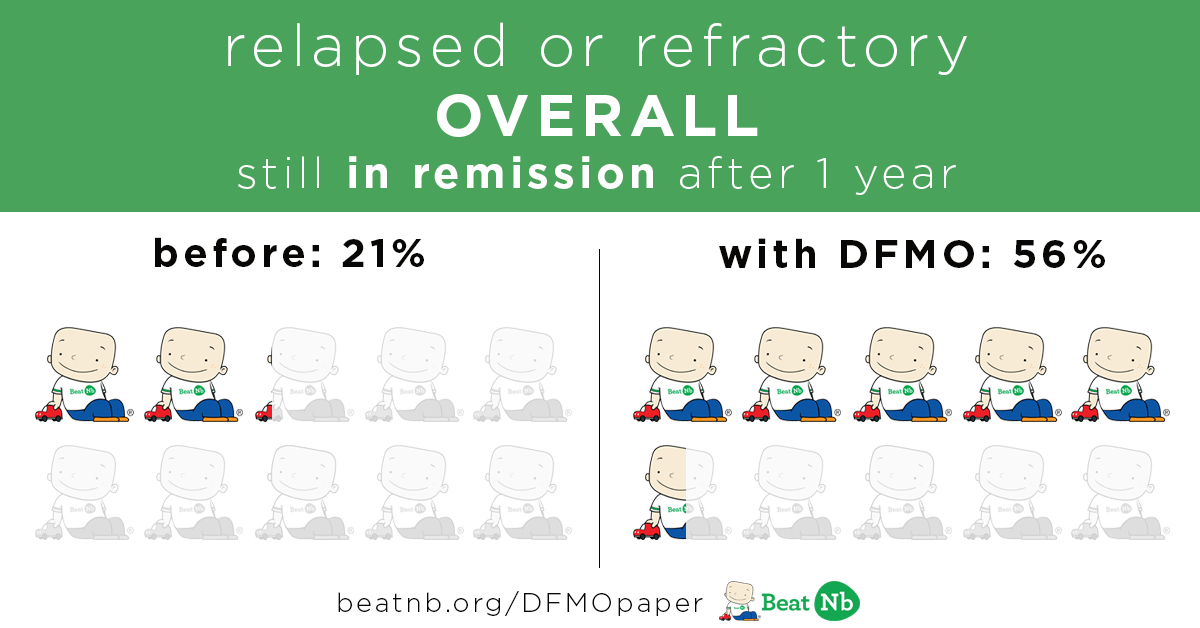
Kids still in remission after 1 year:
Before - 21% With DFMO - 56%
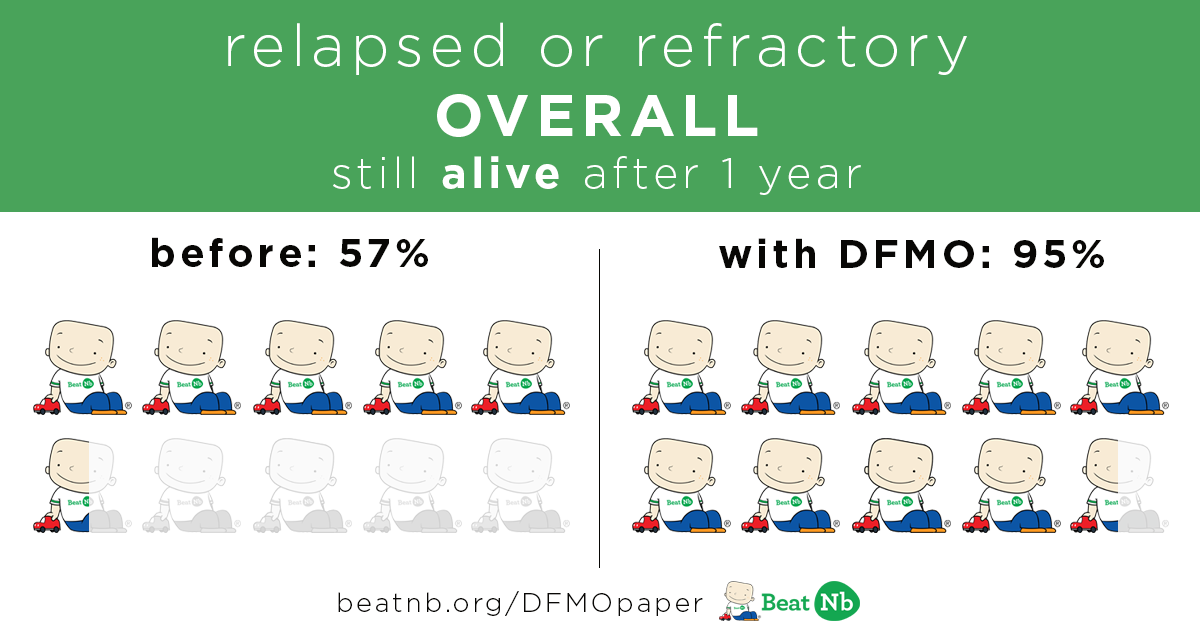
Kids still alive after 1 year:
Before 57% With DFMO - 95%
Kids still in remission after 4 years:
Before - 6% With DFMO - 44 %
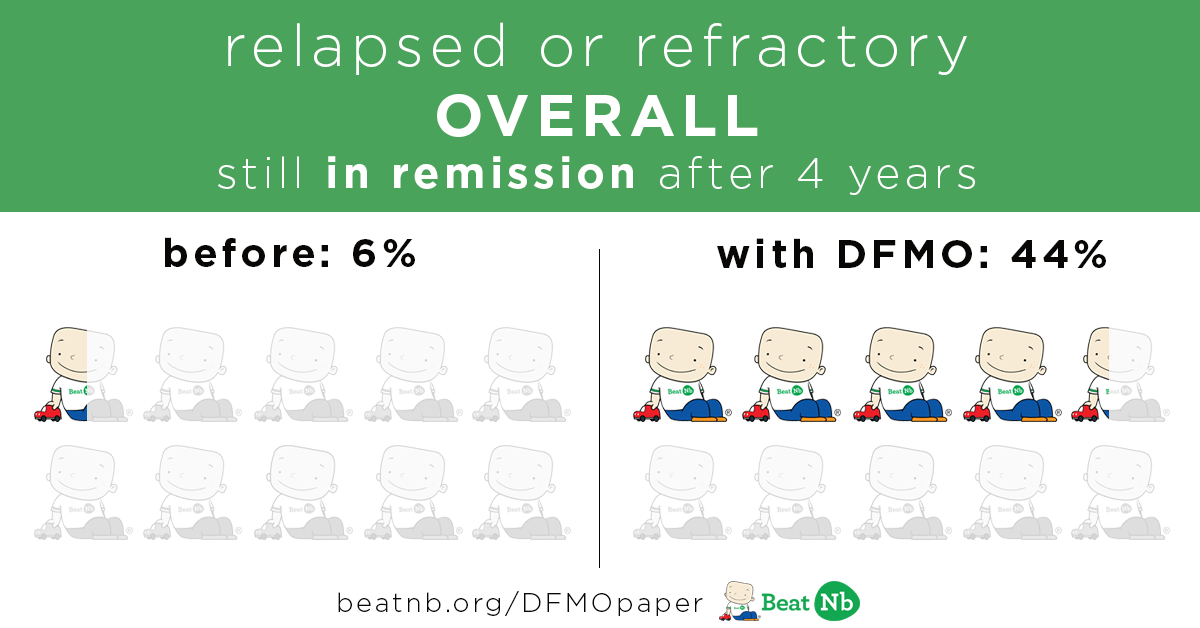
Kids still alive after 4 years:
Before - 20% With DFMO - 62%

Now let's take a look at the subgroup of kids whose tumors were MYCN amplified
MYCN+ Kids still in remission after 1 year:
Before - 13% With DFMO - 71%
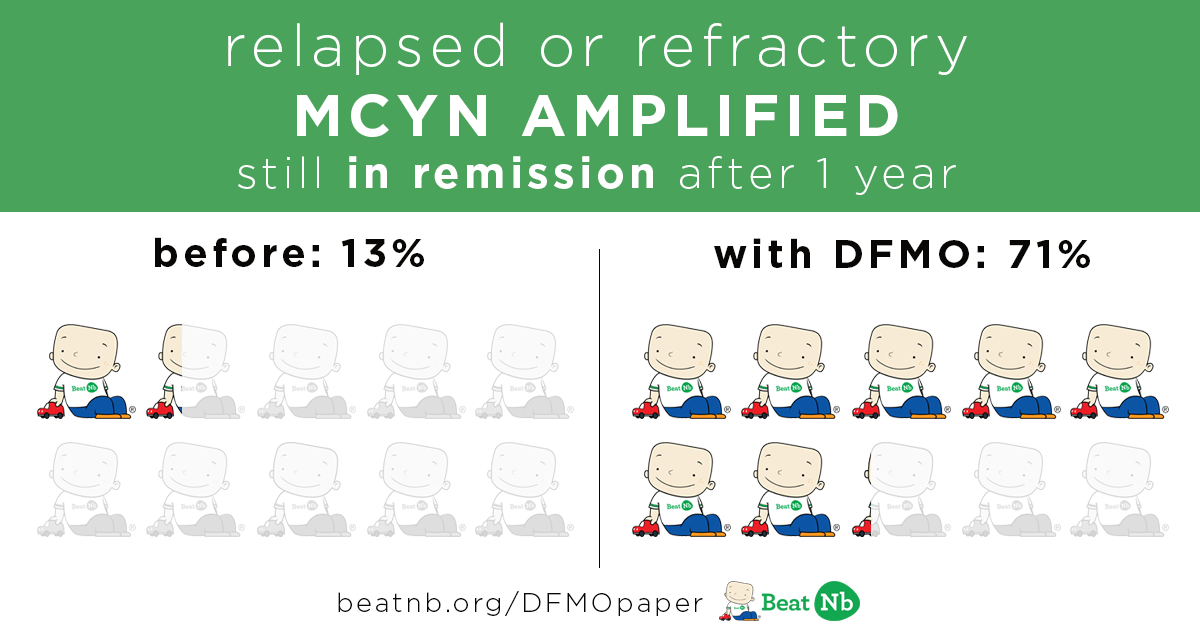
MYCN+ Kids still alive after 1 year:
Before - 30% With DFMO - 86%
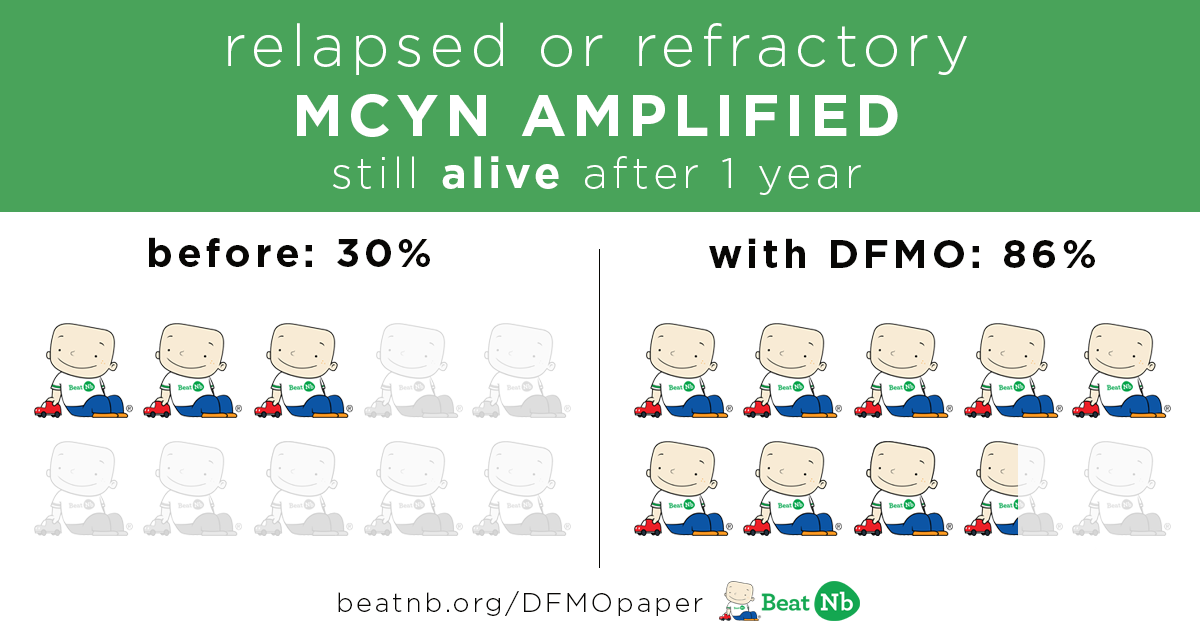
MYCN+ Kids still in remission after 4 years:
Before - 0% With DFMO - 57%
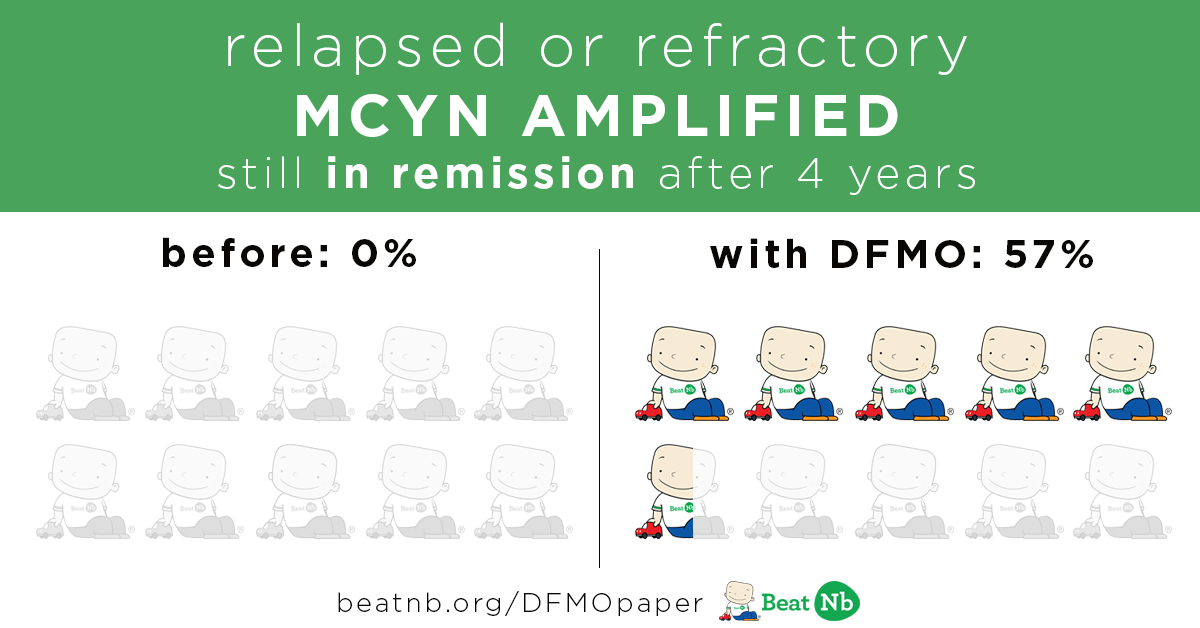
MYCN+ Kids still alive after 4 years:
Before - 0% With DFMO - 69%
These results - including toxicity profile, increases in survival, and sustained remissions - show the potential for a dramatic increase in survival for a group of patients that have historically done poorly.
Let us further break down - and define - the characteristics that make up each of these patient groups reported on.
What are the categories of patients covered in the paper?
Stratum 1:
This includes patients who were diagnosed with neuroblastoma and enrolled on the standard treatment for their disease where they lived. These kids then completed their upfront therapy and were 'done' with treatment. This is normally where kids stop all treatment and hope it works (66% of these kids make it two years without dying or relapsing). Instead? These stratum 1 patients started taking DFMO for two years with the goal of preventing relapse and increasing that 66% figure.
Stratum 2:
This includes patients who had previously had their cancer come back - or their disease did not respond to the upfront therapy - and then were able to get back into remission. These stratum 2 kids were also given DFMO for up to two years once they were in remission to see if you could keep their disease from coming back.
Historical Survival Curves for kids who are NED for Stratum 1
Before we get to defining the historical percentages of survival, let's first outline what we are measuring to calculate survival. The goal of this study was to see if the 2 year EFS and the 2 year OS could be improved upon.
EFS: Event Free Survival - this tells you the percentage of kids at two years who have not died and who have not relapsed with new disease
OS: Overall Survival - this tells you the percentage of kids at two years who are still alive. This includes kids who are still alive but may be battling a new relapse.
Stratum 1 Historical numbers:
There are numerous upfront therapies that you could enroll on (the European protocol, a protocol out of Sloan in New York, or the current standard in the US which is from a study called ANBL0032). Currently, the best published results ever reported on upfront neuroblastoma survival come out of that ANBL0032 trial - it is the current 'gold standard' in the US.
What is remarkable is out of the 100 kids who enrolled and could be evaluated in Stratum 1 for the DFMO study, 81 of them did so directly after finishing their enrollment in the ANBL0032 study. So what are the survival statistics for the current gold standard?
Prior Best 2 year EFS
2 year EFS - From the start of immunotherapy was:
66% at 2 years
Prior Best OS
2 year OS - From the start of immunotherapy was:
86% at 2 years
So prior to DFMO - in the history of mankind - the best published results for 2 year survival for kids with high risk neuroblastomas (HRNB) showed that 66% of them could get 2 years out from the start of antibody before relapsing or dying - and that 14% of the kids would be dead within 2 years of starting antibody.
DFMO Comparison
At this point - it is critically important to explain the next step in the process. While we have the 2 year EFS/OS numbers for DFMO it is not correct to directly compare them to the numbers above.
Why?
It is at this point in the process where we need to explain how you can not - and this paper is not - doing a direct comparison from the numbers above (66% - 86%) to this study. Let me explain why.
The above ‘gold standard’ results looked at 2 year survival from the first dose of antibody given to the kids in the prior study. They were measuring if the addition of an anti-GD2 antibody would improve survival so their start point was the start of immunotherapy.
For this new paper looking at DFMO maintenance therapy lets look at the subset of 81 out of the the 100 patients from Stratum 1 who immediately prior to enrolling on the DFMO study were enrolled in the very 'gold standard' of care study we mentioned above.
These 81 kids were not 'like' those patients - they were actually on that prior trial - and thus provide a remarkable contemporaneous control group. As a result - this subset of 81 patients was used to perform an analysis of patient risk variables that were matched to prior ANBL0032 study kids - including time from diagnosis to enrollment on the study, initial disease stage, MYCN status, age at diagnosis and response to induction therapy.
What does this mean?
What they did was discover that the median time from the start of antibody therapy in the prior study (when they started to measure the EFS/OS that resulted in the 66% and 86% above) to the time that these kids took their first dose of DFMO was 7.2 months.
Sadly, in that 7.2 month period from when they began measuring survival to the time these stratum 1 kids would have gone on DFMO you have some kids dying and some kids relapsing. It would unfairly inflate the results if you excluded that time frame so the statisticians looked at the results that were published from the ‘gold standard’ study to determine the following:
What was the EFS/OS of that study 7.2 months out from the start of immunotherapy?
EFS 66%
7.2 months later - after accounting for the relapses/deaths - they are now comparing the DFMO kids to an EFS of 75%
OS 86%
7.2 months later - after accounting for the relapses/deaths - they are now comparing the DFMO kids to an OS of 91%
What does all of this mean?
Since the best survival numbers ever published started measuring survival on the day that kids starting taking antibody - and on this new study the average kid started taking DFMO 7.2 months after their first dose of antibody - you have to account for all of the relapses and deaths of kids that took place in that 7.2 month time period while on antibody. By getting the EFS & OS from their curve at this time point you are able to make a direct comparison.
So let's get to it.
Stratum 1 patients survival DFMO vs. NO-DFMO (81 patients subset)
2 year EFS with DFMO: 86% (vs. 75% for those without DFMO after the 7.2 month adjustment)
2 year OS with DFMO: 97% (vs. 91% for those without DFMO after the 7.2 month adjustment)
Stratum 2
As previously mentioned the stratum 2 patients were very different than the patient population in stratum 1 which “... includes patients who had previously had their cancer come back - or their disease never responded to therapy - and then somehow were able to get back into remission. These kids were then given DFMO for up to two years to see if you could keep their disease from coming back.”
Historical Survival for kids who are NED for Stratum 2
Treatment for relapsed or refractory neuroblastoma involves many different therapies and approaches (chemotherapy, radiation, MIBG, antibodies, etc..) offered in a phase I or phase II setting or simply as an off-label use of a drug combination in an effort to find something that is effective. At best these treatments have had modest response rates which are sadly followed by high rates of relapse “generally 80-90% within two years”.*
With so many different and highly toxic therapies having such a low response rate this patient population seemed an ideal place to try and increase survival with a novel and low toxicity approach in light of the heavy pretreatment burden these kids have to endure.
A review of recent studies published in 2017 determined the historical rates of progression free survival (PFS) and overall survival (OS). They are listed below and not surprisingly the outcomes were worse for those kids with MYCN amplification in their tumors.*
*London, W. B. et al. Historical time to disease progression and progression-free survival in patients with recurrent/refractory neuroblastoma treated in the modern era on Children’s Oncology Group early-phase trials. Cancer123, 4914–4923, https://doi.org/10.1002/cncr.
PFS
- 1 year: 21%
- 4 year: 6%
OS
- 1 year: 57%
- 4 year: 20%
MYCN amplified PFS
- 1 year: 13%
- 4 year: 0%
MYCN amplified OS
- 1 year: 30%
- 4 year: 0%
Stratum 2 Results
There were 39 kids who enrolled in stratum 2 who had received various upfront and relapse therapies. There is a median follow up time of 3.7 years (range of 2.1 - 5.8 years).
First we’ll take a look at the 2 year EFS/OS for all stratum 2 patients and then we’ll dive deeper to compare the refractory to relapsed patients in this group.
EFS for ALL stratum 2 patients
2 year: 54%
OS for ALL stratum 2 patients
2 year: 84%
-----
EFS for RELAPSED stratum 2 patients
2 year: 35%
OS for RELAPSED stratum 2 patients
2 year: 80%
--------
EFS for REFRACTORY stratum 2 patients
2 year: 68%
OS for REFRACTORY stratum 2 patients
2 year: 89%
As you can see the overall EFS/OS numbers are better for all the kids on DFMO at 2 years than the previously reported one year survival figures! Even the relapsed kids EFS/OS is better at two years than historical 1 year survival.
However, the figure that really jumps out at you from this paper is the EFS of 68% and the OS of 89% at two years for the kids with refractory disease.
What does all of this mean? What is next?
With few exceptions the standard of care for neuroblastoma - be it upfront therapy at diagnosis or with salvage therapy post relapse - has not changed dramatically in the last twenty years save for the introduction of an anti-GD2 antibody therapy. And while there was a demonstrated increase in two year EFS and OS with this therapy the cost and toxicity are not to be ignored. More importantly, the latest update provided on the results of the ANBL0032 study that demonstrated this increase in survival is showing that at 4 and 5 years out from the start of antibody the difference seen in EFS/OS for kids who did - and did not - get antibody are becoming no longer statistically significant.
Therefore, it is imperative that something be done to:
-
Get more kids into remission
-
Keep more kids there
-
Work to decrease the toxicity currently used to achieve goals 1 & 2 above
Given those needs and the encouraging results from this study the following steps are underway with the development of DFMO.
-
After meeting with the FDA another phase II confirmatory trial was opened. The goal was to replicate the results seen in this trial with a shorter time to enrollment along with further stratification of patients (COG, Sloan, Europe,etc…). This trial will be finished enrolling patients in 2018 and with the study endpoints being two year EFS and two year OS the results will be completed by the end of 2020.
-
In the study that was published - as well as in the confirmatory study mentioned above - DFMO is given after patients are finished with their anti- GD2 antibody. Sadly, about 14% of the patients who take this antibody relapse while taking it. Therefore, a trial was opened three years ago (September of 2015) for kids newly diagnosed with NB that is randomizing kids onto DFMO when they start antibody. Half of these kids will be given DFMO during antibody - the other half will start it at the end of antibody. This study is giving DFMO during antibody in order to see if that 14% relapsed figure could be reduced.
The goal is to see if DFMO can keep more kids in remission while finishing upfront therapy. This would then allow these kids - who would have otherwise relapsed - to continue taking DFMO for the two year maintenance period.
Mind if we hang out
in your inbox?
We only email once or twice a month, always relevant to how we're working to beat childhood cancer for every child, everywhere.
Thank you for subscribing!
Something went wrong.